PROJECTS
PROJECTS
Research Area A : Microglia

Research Area B : T cells

Research Area C : Human translation

Research Area Z : Core project (animal models)
Research Area A : Microglia
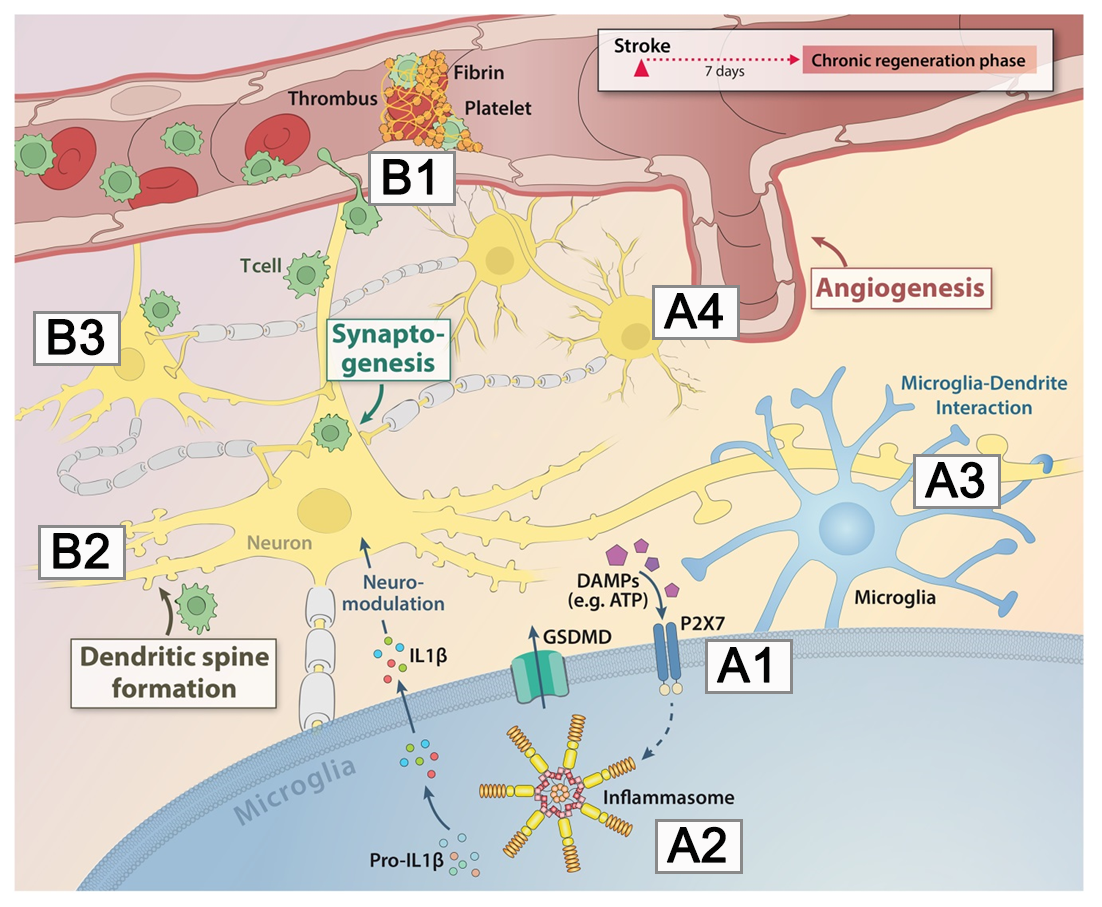
In this project, we want to study the effect of so-called “danger signals” or “dangerassociated molecular patterns” (DAMPs) as activators of the local immune response in stroke.
read more
The goal of this proposal is to develop new treatment strategies for enhancing neuronal repair using nanobodies against danger signal receptors (e.g., P2X7). By targeting danger signals, we hope to modulate the stroke-induced sustained immune response, thereby improving functional recovery and neuronal plasticity.
A2: Inflammasomes in post-stroke regeneration
This project will investigate the role of cerebral inflammasome activation in chronic neuroinflammation after ischemic stroke.
read more
We will particularly investigate the contribution of different inflammasome sensors for chronic neuroinflammation and test üharmacological approches for inhibition of inflammasome activation. Furthermore, we will analyze the non-immunological role of inflammasome in enruonal plasticity via localized cell death and dendrite retraction involved in post-stroke neuronal plasticity.
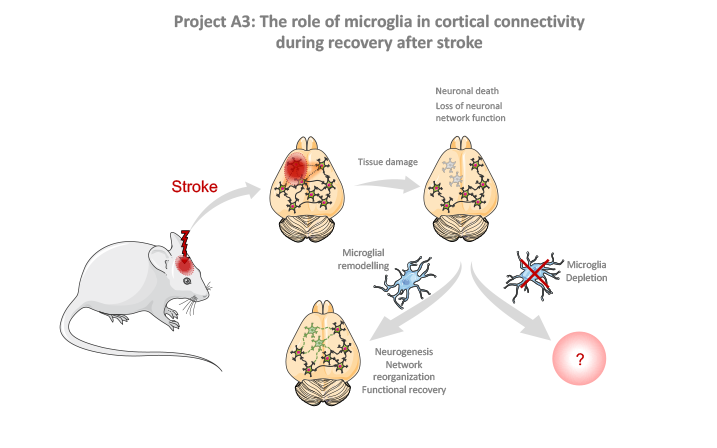
A3: The role of microglia in cortical connectivity during recovery after stroke
The aim of this project is to test the contribution of chronic microglial activation after stroke on post-stroke functional recovery.
read more
We will adopt complimentary approaches to deplete microglia either in timed intervals or chronically after stroke and will investigate the effect of microglial depletion on functional recovery. For this, we will combine multiple readouts for neuronal plasticity, beahvior and neuronal network function to comprehensively assess post-stroke recovery.
A4: Immune responses controlling post-stroke angiogenesis and microvascular integrity
The overall objective of this project is to investigate the role of microglia and T cells in controlling microvascular integrity during angiogenesis in post-stroke recovery
read more
by investigating the following aims: AIM 1: Characterize the effects of microglia on microvascular integrity and post-stroke angiogenesis, AIM2: Characterize the effects of T cells on post-stroke microvascular remodeling, AIM 3: Identify molecular targets of inflammation-mediated microvascular remodeling
Research Area B: T cells
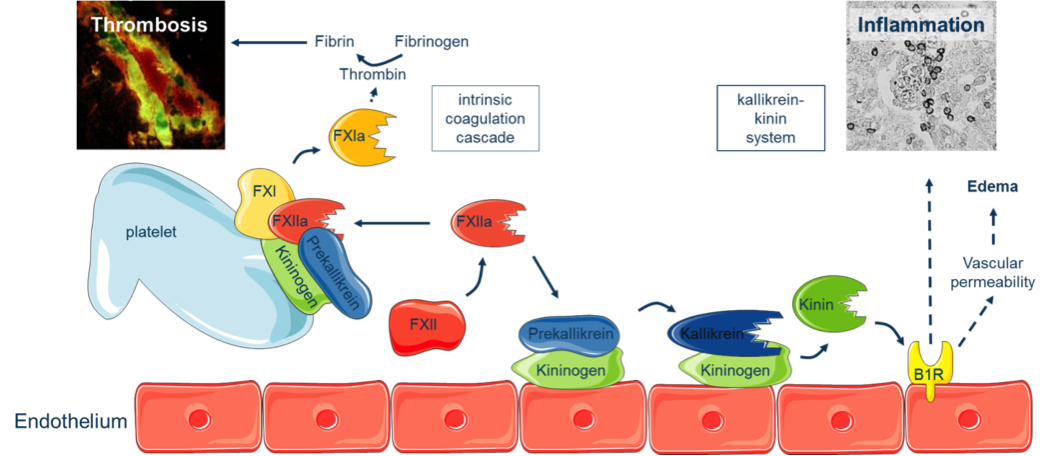
B1: Kallikrein-kinin system-mediated thrombo-inflammation as a novel target to improve long-term stroke recovery
Aim of this project is to screen members of the Kallikrein-Kinin-System (KKS) and different immune cell subsets regarding their relevance for long-term stroke recovery.
read more
We will use readily available transgenic mouse strains carrying mutations in the KKS, or pharmacological inhibitors interfering with the KKS, and mice with selective defects in different immune cell subpopulations. In these animals stroke will be induced and long-term functional recovery, neuronal plasticity and angiogenesis will be analyzed.
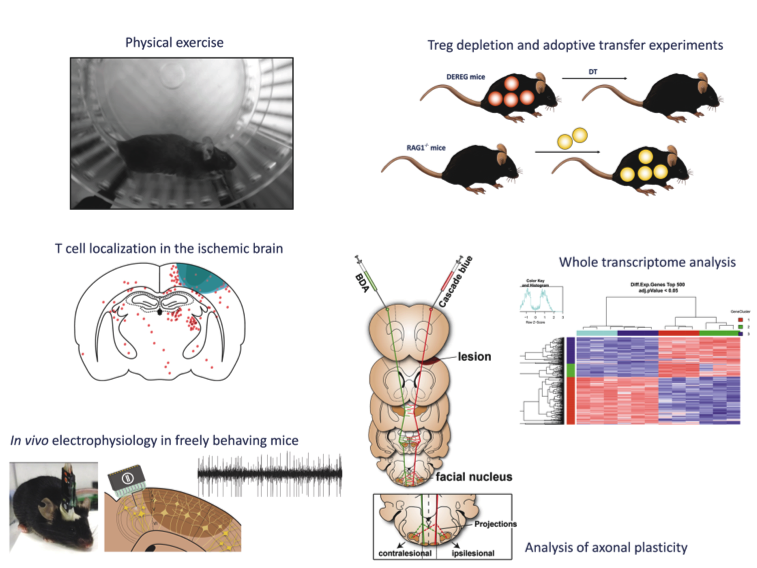
B2: The role of T cells in exercise-induced recovery after stroke
The project aims to clarify the role of T cells for exercise-mediated long-term recovery following ischemic stroke by carefully dissecting the interplay between exercise, T cells and neuronal plasticity following stroke.
read more
For this purpose we will analyze the effect of exercise on T cell localization in ischemic brain and on the functional and molecular phenotype of T cells as well as the T cell-mediated exercise effects on neuronal plasticity, i.e. axonal sprouting and neuronal excitability. Overall, we aim to provide the basis for future recovery-enhancing therapies that modulate identified molecular targets – so called exercise mimetics.
B3: Decoding neuronal expression networks governed by neuro-immune interactions during stroke recovery
The overall objective of this project is to decipher immune-mediated neuronal programs that determine stroke recovery.
read more
To identify key molecular mechanisms underlying neuronal cell death in early stages we will use mice transgenic for a bacterial artificial chromosome (BAC) that encodes the EGFP-tagged ribosomal protein L10a driven by a cell-specific promoter. Using the transgenic mouse line Glt25d2 bacTRAP will allow us to affinity purify polysomal mRNAs (translatome) specifically from cortical layer V neurons following stroke. To identify neuroregenerative pathways in the neuronal translatome that are driven by T cells or microglia we will selectively deplete CD4 cells, CD8 cells or microglia.
Research Area C: Human translation
C1: Linking functional immune profile and ischemic lesion characteristics in human stroke
The aim of Project C1 is to elucidate the dynamics of stroke-related changes in peripheral immune signatures and functional properties of immune cells, and to study these changes in relation to stroke subtype and to the characteristics of ischemic stroke lesions.
read more
This project will address immunometabolism as potential driver of immune-mediated neuronal damage, and purinergic signaling as a potential mechanism underlying stroke-induced immune suppression. These properties will be analysed in a prospectively acquired bicentric cohort of patients with ischemic stroke at different time points.
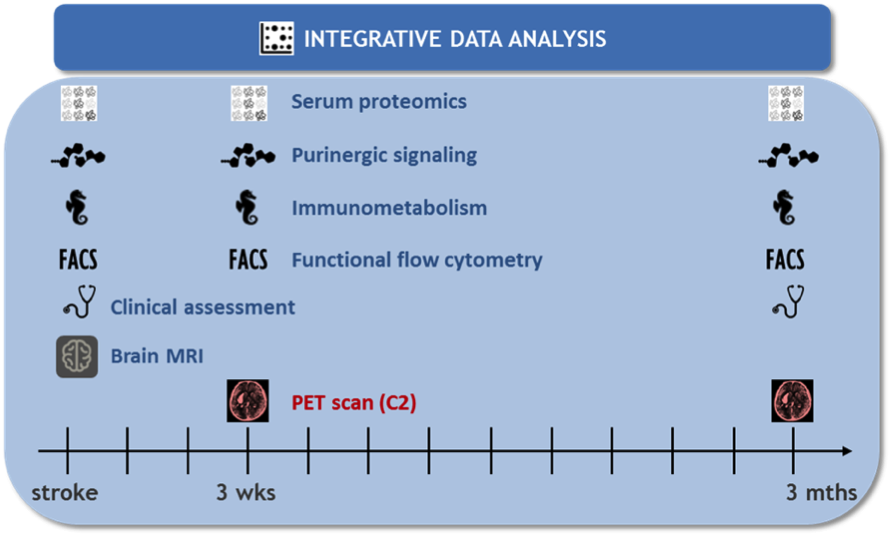
C2: Microglia-PET as a surrogate marker for post-stroke neuroinflammation
We will perform serial [18F]-GE-180 PET imaging along with MR imaging, immune cell profiling in blood, clinical and laboratory assessments in 36 patients with acute ischemic stroke
read more
to (1) define the characteristics and determinants of microglia activation after human stroke; (2) correlate microglial activation with the profile of inflammatory markers and circulating immune cells; (3) correlate microglial activation with infarct evolution, secondary neurodegeneration, and stroke outcome; and (4) develop criteria for selecting patients for future immune intervention trials. We will further compare the characteristics of microglial activation patterns in human stroke with those of experimental stroke in mice.
Research Area Z: Core project (animal models)
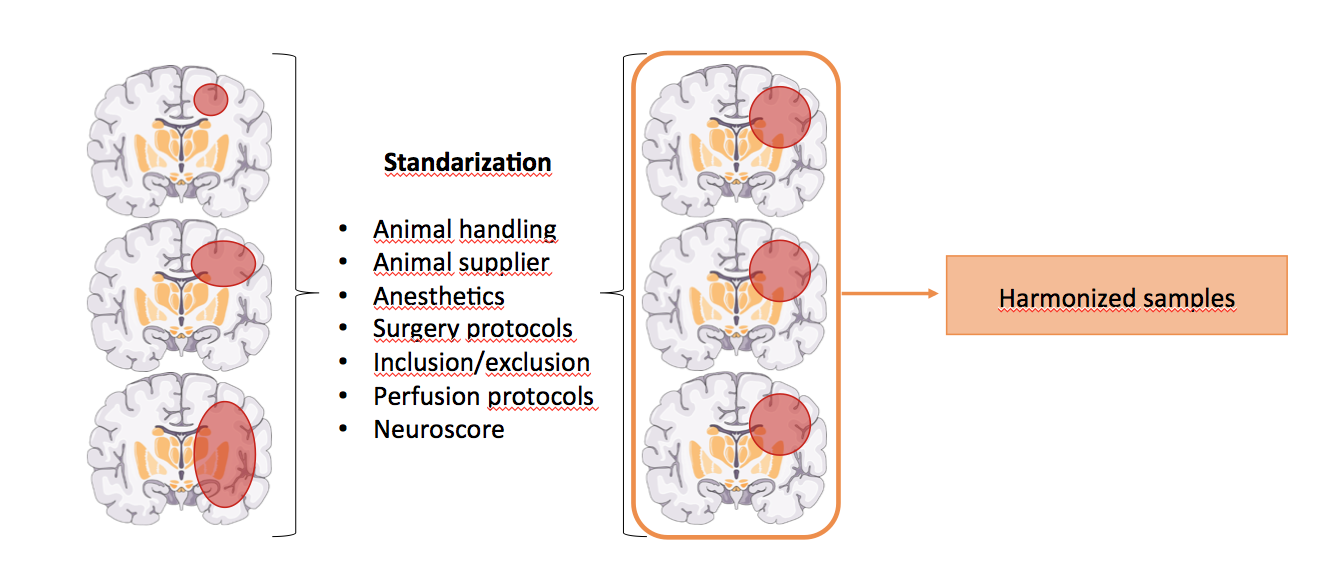
Z2: Standardization of animal models and outcome parameters
The overall goal of this project is to define new standards in performing experimental research in stroke recovery and set the stage for a preclinical multicenter study in the second funding phase.
read more
This will be achieved by first defining the most appropriate lesion models and outcome parameters to study stroke recovery, then define SOPs for these techniques, train collaborating laboratories of the research groups on these SOPs and finally prepare the infrastructure for a preclinical multicenter RCT. Additionally, this core project will provide a pre-publication cross-check of experimental in vivo methodology and coordinate an internal peer review of manuscript reporting results of the research group.
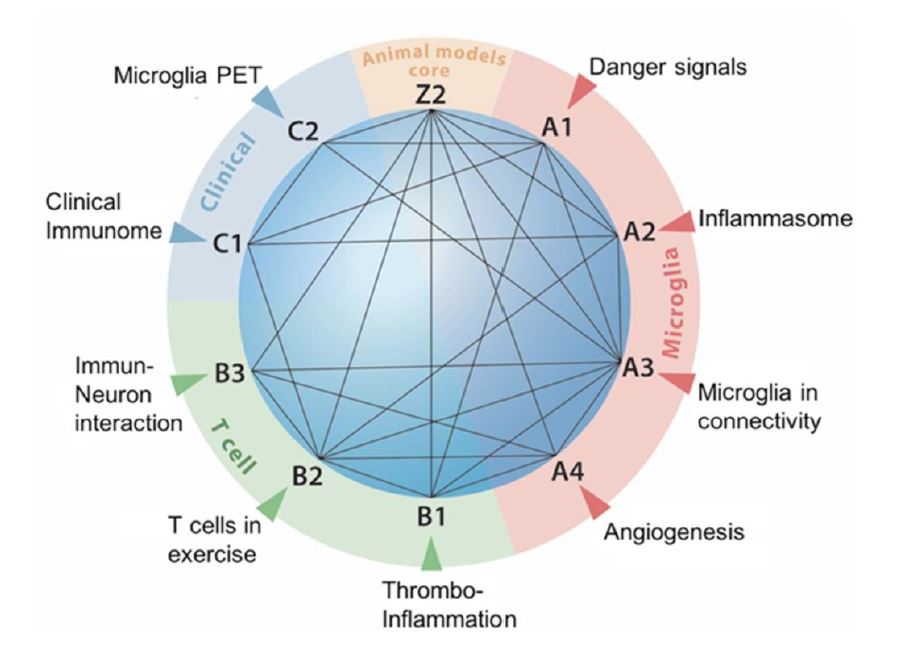
Research areas and interaction of projects
Our research group will investigate mechanisms of
regeneration and neuronal plasticity in the chronic phase after stroke with a focus on inflammatory mechanisms
driven by microglia and T cells. Accordingly, research Area A will investigate microglia-brain interactions while T
cells will be in the focus of research Area B. These two preclinical research areas will be complimented by two
clinical observatory studies in research Area C and a core project (Z2) for standardization of animal models and
outcome parameters.
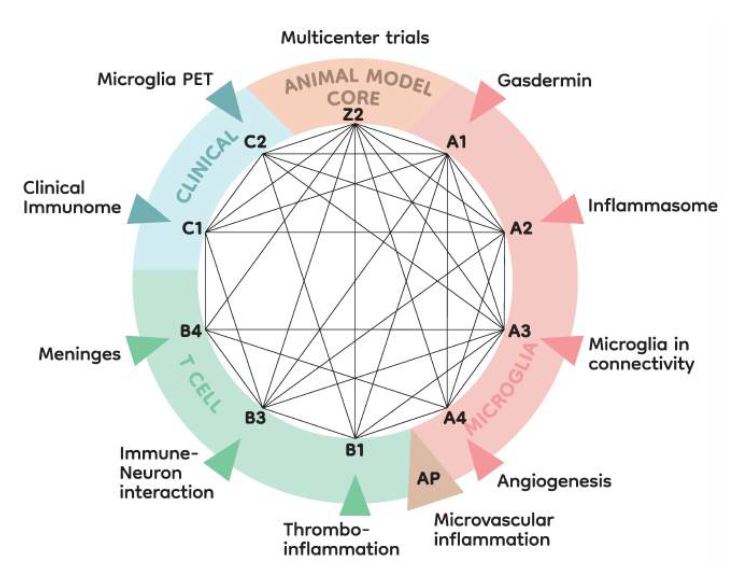
In the second funding period, the overall structure of our consortium was maintained. We kept the three research areas: microglia (research Area A), T cells (research Area B) and clinical studies (research Area C). In addition, the Z02 project will conduct two pRCTs while maintaining the animal models platform.
Research areas and interaction of projects. In continuation of the structure of ImmunoStroke established in the first funding period, Area A will investigate microglia-brain interactions while T cells will be the focus of research Area B. These two preclinical research areas will be complemented by two clinical observatory studies in research Area C and a core project (Z2) for pRCTs and animal models platform. After positive evaluation, a topically related project (AP) will be associated.